we discover ...
Privacy Policy
You are welcome to change your privacy preferences here.
Find further details in our Privacy Policy
ABOUT US Biotracing
ABOUT US
The economic hub Bremerhaven opens a gate to the world which is exactly the cosmopolitan openness mir|detect needs to thrive.
Cancer is an international topic.
Many types of cancer are not researched enough yet.
mir|detect creates reliability and certainty not only in the section of testicular cancer.
mir|detect: Biotechnology from Bremerhaven.
TEAM Biotracing













HISTORY Biotracing
COMPANY HISTORY
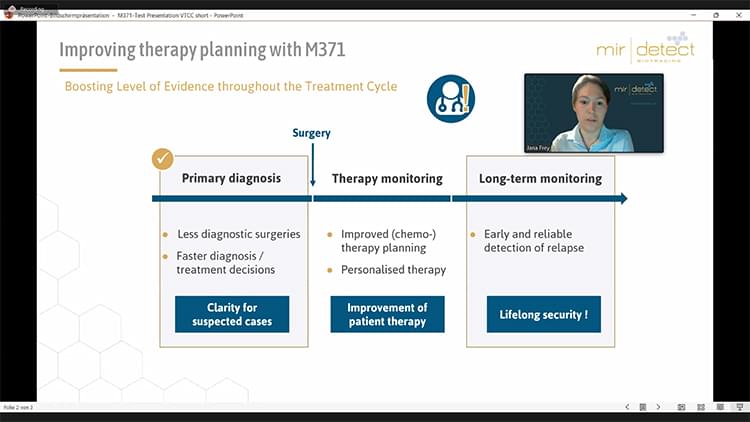
M371-Test CE-certified according to IVDR
M371-Test validated on two new qPCR-platforms
Study on the effectiveness of the M371-Test in follow-up monitoring published.
Exclusive Distribution Agreement for Europe
with Gold Standard Diagnostics
Presentation of
M371-Test at the DGU congress in Stuttgart
ISO 13485 certification of mir|detect's QMS
M371-Test CE-certified according to IVDD
Finalist of the
EIT Health Headstart Förderprogramms 2019
Nominated for the
ACHEMA-Gründerpreis 2018
Presentation of the biomarker miR371a at the
9th Copenhagen Workshop on Testicular Germ Cell Cancer
Awarded
Sience4Life Cup 2017
Finalist of the
BioVaria-Kongress 2017 in Munich
Awarded
Venture Lounge 2016
Foundation
mir|detect GmbH
M371 Project started
at the University of Bremen
NEWS Biotracing
Archive | 2021
4th of April 2024
CE CERTIFICATION
Successful CE2797 certification of the M371-Test according to IVDR
M371-Test developed by mir|detect for the detection and monitoring of testicular germ cell tumors has been approved in accordance with Regulation 2017/746 IVDR since April 2024, and thus receives one of the first IVDR certificates in the EU.
The new EU regulation significantly tightens the regulatory requirements compared to Directive 98/79/EC IVDD and ensures greater safety for patients and users in the use of in-vitro diagnostics.
The IVDR certification of the M371-Test guarantees that the scientific validity as well as the analytical and clinical performance has been extensively tested and confirmed by a notified body.

3rd of March 2024
NEW CYCLERS
Outstanding diagnostic performance of the M371-Test on new qPCR platforms
mir|detect has validated the M371-Test for the detection of testicular germ cell tumors for use on two new qPCR platforms:
- AriaDx (Agilent)
- QuantStudio 5 (ThermoFisher Scientific)
The M371-Test offers the same excellent diagnostic performance on both devices as on the LightCycler 480 II, increasing accessibility for laboratories and thus the number of patients who benefit from the M371-Test.
Furthermore, mir|detect is working hard on the validation of the M371-Test on the LightCycler PRO (Roche) and the CFX96 (Bio-Rad) to make M371-Test accessible to many patients and users.
15th of January 2024
FOLLOW-UP MONITORING
Excellent performance of M371-Test in follow-up monitoring confirmed
The M371-Test provides you with unparalleled diagnostic accuracy, offering a groundbreaking approach to the management of testicular cancer. A groundbreaking study confirms the excellent performance of mir|detect's M371-Test in detecting recurrences after testicular germ cell tumors.
Early detection of recurrent disease is crucial in the follow-up of patients with testicular germ cell tumors. In a recently published study, serum levels of miR-371a-3p were monitored in 258 patients using mir|detect's M371-Test. During the follow-up, 39 relapses occurred and elevated miRNA-levels were detected in all cases which corresponds to a sensitivity and specificity of 100% and 96.3%, respectively.
Thus, these results underline the suitability of mir|detect’s M371-Test for the detection of recurrences and the test represents a major advancement in the follow-up monitoring of testicular cancer patients.
The paper was published in Clinical Cancer Research and is now available online: https://doi.org/10.1158/1078-0432.ccr-23-0730
12th of May 2023
DISTRIBUTION MEETING
Meeting with Gold Standard Diagnosics in Bremerhaven
On May 12, 2023, we received a visit from a team of our exclusive distribution partner, Gold Standard Diagnostics, at our premises in Bremerhaven.
We are proud to continue the path we started in November 2022 with this well-connected and established partner throughout Europe, and we look forward to further intense and fruitful collaboration.

April 2023
VENTURE CAPITAL MAGAZIN
New Gold Standard


21st - 23rd of September 2022
DGU HAMBURG
74. Congress of the German Society of Urology
Over three days mir|detect presented the test M371 at the congress of the German Society of Urology at the company's own booth and was available for interested questions.
Prof. Dr. Klaus-Peter Dieckmann shared his latest research findings on miRNA-371a with colleagues as part of the scientific program.



19th - 20th of May 2022
UROBAY LINDAU
Testicular Cancer Awareness Foundation
mir|detect was presenting M371 at the M371-both at the UroBay in Lindau.



20th - 21st of April 2022
VIRTUAL TESTICULAR CANCER CONFERENCE
Testicular Cancer Awareness Foundation
mir|detect was happy to provide a platinum sponsorship for this conference.
Jana Frey ( mir|detect - product management ) presented M371 to all participants of the virtual meeting.

7th of October 2021
PRESS RELEASE:
BioVendor | Laboratorni medicina a.s. | Czech Republic
M371 is now available through BioVendor in Brno, Czech Republic.
Click here for the press release
15th to the 18th of September 2021
DGU CONGRESS STUTTGART
German Association of Urology
mir|detect presents M371 at the annual congress of the German Association of Urology. Interested medical staff had the opportunity to directly ask questions to our team.



15th of June 2021
PRESS RELEASE:
GeLaMed | Eurofins Laborbetriebsgesellschaft Gelsenkirchen GmbH
M371 is now available at the Eurofins Laboratory in Gelsenkirchen (GeLaMed). M371 can be ordered through your urologist.
Click here for the press release

CAREER Biotracing
FOR JOB OFFERS PLEASE VISIT OUR GERMAN SITE
We've embedded content from OpenStreet Map here. As OpenStreet Map may collect personal data and track your viewing behaviour, we'll only load the map after you consent to their use of cookies and similar technologies as described in our privacy policy.