
M371-Test | The future of testicular cancer diagnostics
The innovative M371-Test from mir|detect detects the tumour-specific miR-371a-3p by means of a minimally invasive blood draw.


Background
Biotracing
Previous standard diagnostics
Testicular cancer is the most common cancer in men between the ages of 20 and 45. In Europe alone, there are 25,000 new cases per year and around 60,000 new cases per year worldwide.
Due to their low sensitivity and specificity, conventional serum tumour markers do not provide very reliable results, leaving considerable uncertainty.
In addition, patients who have tested positive and undergone surgery are exposed to considerable cytotoxic radiation from computerised tomography scans (CT scans) as part of their follow-up care. Although the alternative use of magnetic resonance imaging (MRI) for imaging avoids radiation exposure, MRI also causes immense costs in the healthcare system. In view of the fact that a testicular cancer patient must be examined repeatedly (on average up to 19 times) over a period of up to 10 years after initial diagnosis, which includes 2 to 4 imaging examinations per year (CT), it is clear that this is associated with a considerable burden on the patient and also on the healthcare system.
Through the use of the M371-Test the use of CT and MRI examinations can be significantly reduced.
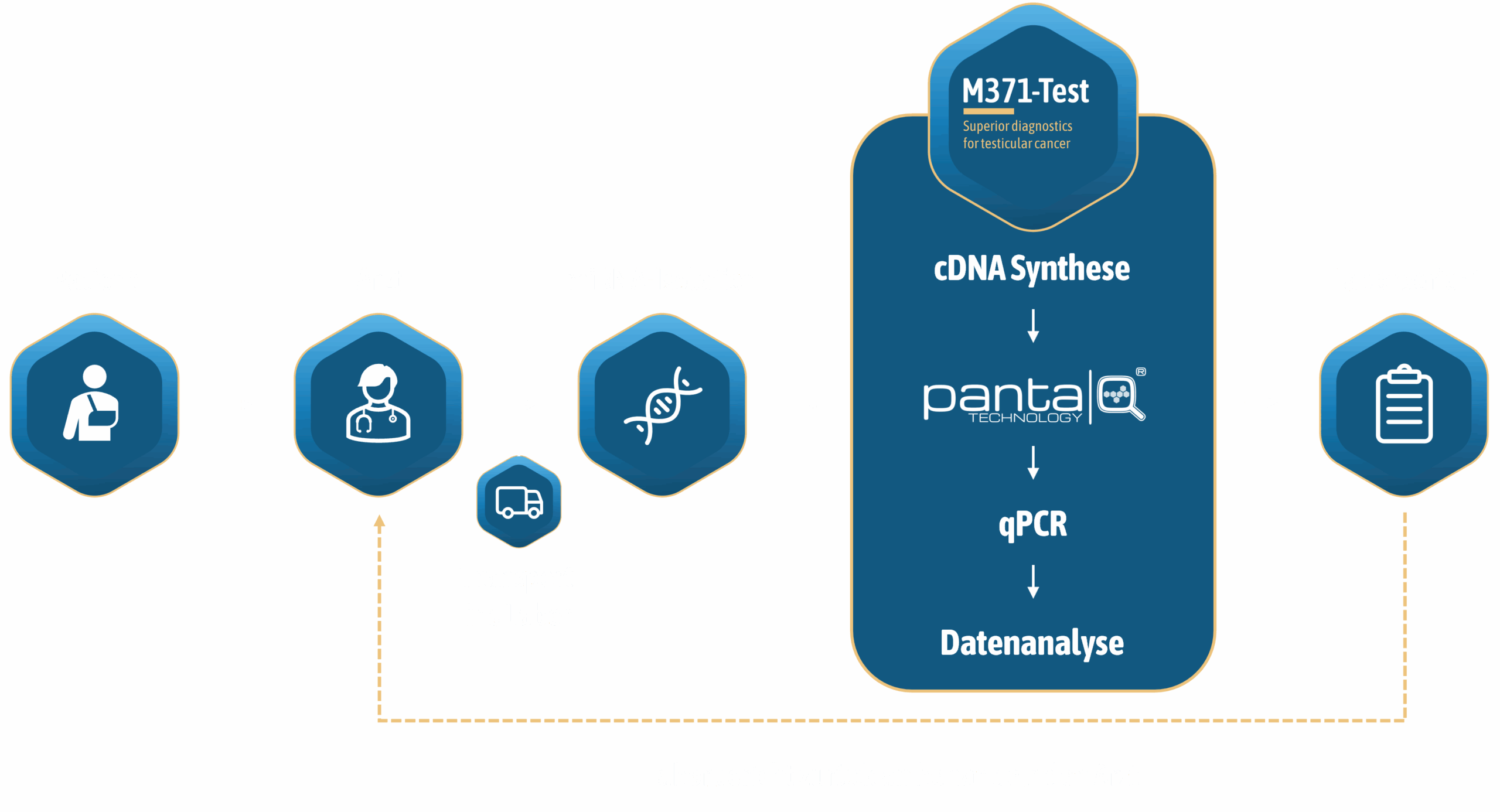
The innovative new development detects the tumour-specific miR-371a-3p in minimally invasive blood samples. The concentration of miRNA in a blood sample is determined by quantitative real-time PCR. The sensitivity and reproducibility of this method for detecting miRNAs, which are often present in low concentrations, is increased by upstream pre-amplification.

Technology
Biotracing
HOW OUR HIGH-PERFORMANCE TUMOUR TEST WORKS
mir|detect has developed the innovative and patented panta|Q®-technology to improve the sensitivity and reproducibility of nucleic acid amplification tests (NAT) in diagnostics. The M371-Test, developed by mir|detect based on the panta|Q®-technology, quantifies the for testicular cancer specific miR-371a-3p which is released by the tumour cells and can be detected in the patient's blood sample. The determination of the concentration of the germ cell tumour-specific miR-371a-3p provides a reliable indication of the presence of testicular cancer. The sensitivity and specificity of this procedure clearly surpasses the informative value of the classic serum tumour markers.

The panta|Q®-technology
The by mir|detect developed and patented panta|Q®-technology sets new standards in sensitivity and robustness in the detection of disease-specific nucleic acids. The technology has extensive patent protection and enables the development of a broad spectrum of nucleic acid-based tests.
Are you interested in using the panta|Q®-technology for your in-vitro diagnostic? Get in touch with us!
Our M371-Test has the potential to improve the diagnosis and treatment process for testicular cancer patients, medical staff and insurance companies at every level. The test enables faster and more individualised treatment decisions to be made and therefore also the possibility of cost savings. By detecting the smallest amounts of tumour-specific miR-371a-3p in a blood sample, the M371-Test can also be used in primary diagnostics in order to make a clear diagnosis more quickly and reliably.
DIAGNOSTICS & therapy success
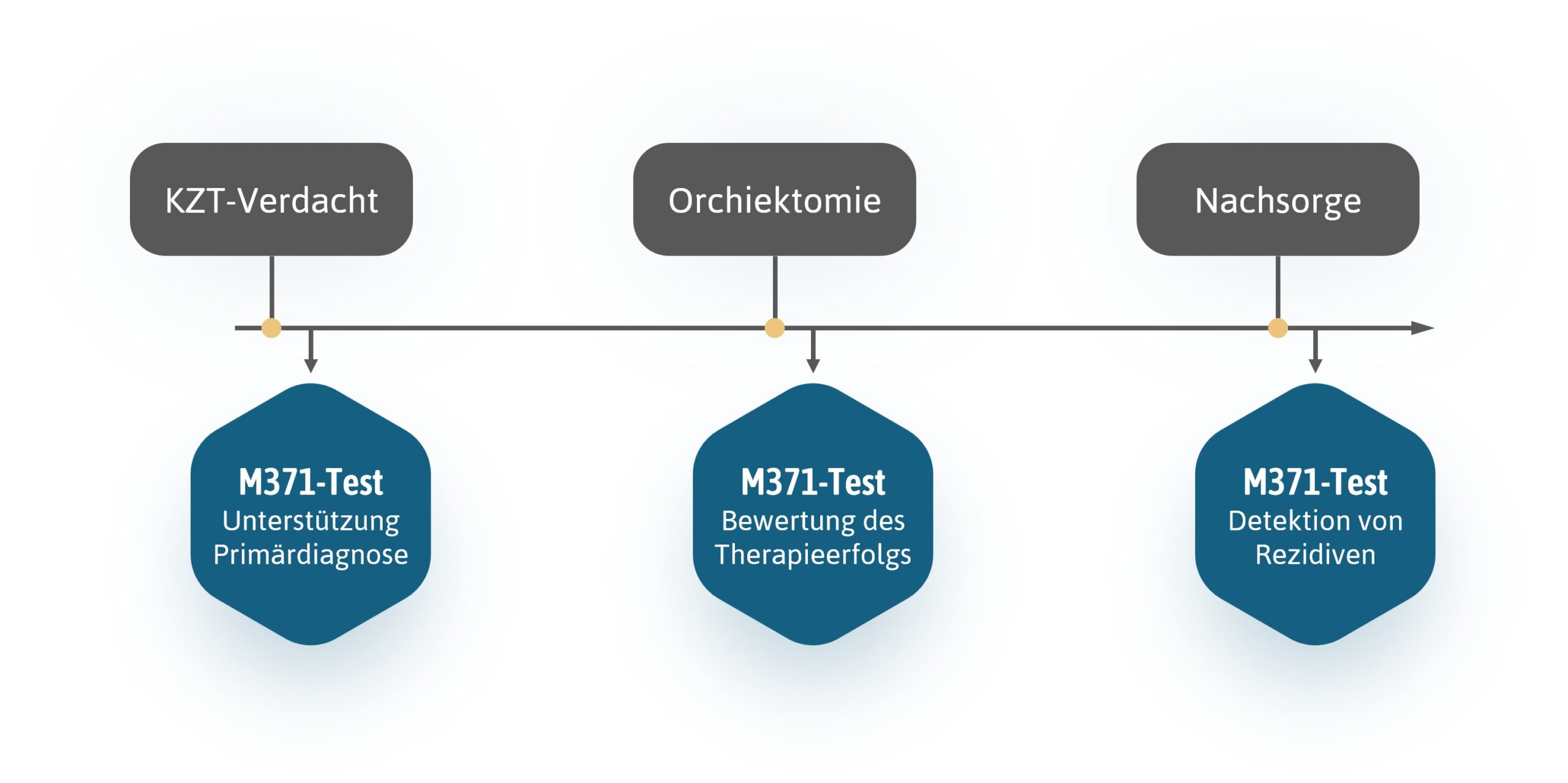
The M371-Test is of great importance not only in primary diagnostics but also in monitoring the success of therapy.

Through the early detection of tumour-specific miR-371a-3p in blood samples, doctors can diagnose testicular cancer more quickly and effectively and monitor the success of therapeutic measures. The M371-Test can indicate remaining tumour masses and enable patients to receive personalised treatment.

Follow-up monitoring
Successful treatment is followed by extensive follow-up monitoring, which can last up to 10 years in the case of testicular cancer. The M371-Test can be used to closely monitor changes in health status.
Due to the high recurrence rate of testicular cancer, long-term follow-up care over a period of up to 10 years is required after successful initial treatment. The M371-Test reliably detects recurrent tumours at an early stage. This enables treatment to be initiated early and thus increases the likelihood of success. By avoiding radiation-intensive examination methods (CT), the risk of secondary cancers can be reduced at the same time.
Application
Biotracing
HOW TO USE the M371 test

Studies & more
Biotracing
Clinical studies on the suitability of the M371-Test in testicular cancer diagnostics
Use in primary diagnostics
The suitability of the M371-Test for primary diagnostics was evaluated in a multicentre clinical study involving 37 centres in several European countries (DE, AT, CH, IT, HU).
Due to its large size with 522 patients (primary diagnosis only) and 258 control subjects, this study is highly informative. The M371-Test achieved excellent results with a sensitivity of 90 % and a specificity of 94 % and proved to be clearly superior to the classic serum tumour markers (Dieckmann et al., 2019).
Use in Follow-up monitoring
On the suitability of the M371-Test A comprehensive, prospective study is available on the follow-up care of testicular cancer patients. In this study, 258 patients were followed up over a period of up to 48 months (median: 18 months). During the observation period, 39 recurrences (15 %) occurred. The result of the M371-Test was positive in all these cases, resulting in a sensitivity of 100 %. In contrast, the serum tumour markers AFP and ß-hCG in combination achieved a sensitivity of only 45 % (Belge et al., 2024).
Performance data for the M371-Test
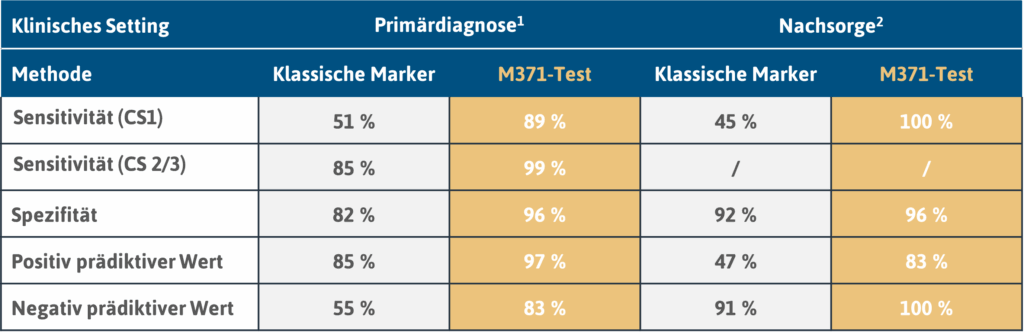
1 n = 522 patients; 258 controls (Dieckmann et al., 2019; https://doi.org/10.1200/jco.18.01480)
2 n = 258 patients (Belgium et al., 2024; https://doi.org/10.1158/1078-0432.ccr-23-0730)
Classic markers: AFP (α-fetoprotein), bHCG (β-subunit of human chorionic gonadotropin) and LDH (lactate dehydrogenase)
Selected publications
SELECTED PUBLICATIONS
Dieckmann KP, Radtke A, Geczi L, Matthies C, Anheuser P, Eckardt U, Sommer J, Zengerling F, Trenti E, Pichler R, Belz H, Zastrow S, Winter A, Melchior S, Hammel J, Kranz J, Bolten M, Krege S, Haben B, Loidl W, Ruf CG, Heinzelbecker J, Heidenreich A, Cremers JF, Oing C, Hermanns T, Fankhauser CD, Gillessen S, Reichegger H, Cathomas R, Pichler M, Hentrich M, Eredics K, Lorch A, Wülfing C, Peine S, Wosniok W, Bokemeyer C, Belge G. 2019. serum Levels of MicroRNA-371a-3p (M371 Test) as a New Biomarker of Testicular Germ Cell Tumours: Results of a Prospective Multicentric Study. J Clin Oncol. 37: 1412-1423. https://doi.org/10.1200/jco.18.01480
Belge G, Dumlupinar C, Nestler T, Klemke M, Törzsök P, Trenti E, Pichler R, Loidl W, Che Y, Hiester A, Matthies C, Pichler M, Paffenholz P, Kluth L, Wenzel M, Sommer J, Heinzelbecker J, Schriefer P, Winter A, Zengerling F, Kramer MW, Lengert M, Frey J, Heidenreich A, Wülfing C, Radtke A, Dieckmann KP. 2024. detection of Recurrence through microRNA-371a-3p Serum Levels in a Follow-up of Stage I Testicular Germ Cell Tumours in the DRKS-00019223 Study. Clin Cancer Res. 30: 404-412. https://doi.org/10.1158/1078-0432.ccr-23-0730
Sohaib SA, Koh DM, Husband JE. 2008. the role of imaging in the diagnosis, staging, and management of testicular cancer. AJR Am J Roentgenol. 191: 387-395. https://doi.org/10.2214/ajr.07.2758
Dieckmann KP, Belge G. 2024. testicular tumour - what are the advantages of the new tumour marker microRNA-371a-3p (M371-Test). Current Urol. 55: 510-519. https://doi.org/10.1055/a-2358-8355
Leão R, van Agthoven T, Figueiredo A, Jewett MAS, Fadaak K, Sweet J, Ahmad AE, Anson-Cartwright L, Chung P, Hansen A, Warde P, Castelo-Branco P, O'Malley M, Bedard PL, Looijenga LHJ, Hamilton RJ. 2018. serum miRNA Predicts Viable Disease after Chemotherapy in Patients with Testicular Nonseminoma Germ Cell Tumour. J Urol. 200: 126-135. https://doi.org/10.1016/j.juro.2018.02.068
Tavares NT, Henrique R, Jerónimo C, Lobo J. 2025. Current Role of MicroRNAs in the Diagnosis and Clinical Management of Germ Cell Tumours. Surg Pathol Clin. 18: 91-100. https://doi.org/10.1016/j.path.2024.08.003
Spiekermann M, Belge G, Winter N, Ikogho R, Balks T, Bullerdiek J, Dieckmann KP. 2015. microRNA miR-371a-3p in serum of patients with germ cell tumours: evaluations for establishing a serum biomarker. Andrology. 3: 78-84. https://doi.org/10.1111/j.2047-2927.2014.00269.x
Dieckmann KP, Radtke A, Spiekermann M, Balks T, Matthies C, Becker P, Ruf C, Oing C, Oechsle K, Bokemeyer C, Hammel J, Melchior S, Wosniok W, Belge G. 2017. Serum Levels of MicroRNA miR-371a-3p: A Sensitive and Specific New Biomarker for Germ Cell Tumours. Eur Urol. 71: 213-220. https://doi.org/10.1016/j.eururo.2016.07.029
Lobo J, Gillis AJM, van den Berg A, Dorssers LCJ, Belge G, Dieckmann KP, Roest HP, van der Laan LJW, Gietema J, Hamilton RJ, Jerónimo C, Henrique R, Salvatori D, Looijenga LHJ. 2019. Identification and Validation Model for Informative Liquid Biopsy-Based microRNA Biomarkers: Insights from Germ Cell Tumour In Vitro, In Vivo and Patient-Derived Data. Cells. 8: 1637. https://doi.org/10.3390/cells8121637
Leão R, Albersen M, Looijenga LHJ, Tandstad T, Kollmannsberger C, Murray MJ, Culine S, Coleman N, Belge G, Hamilton RJ, Dieckmann KP. 2021. Circulating MicroRNAs, the Next-Generation Serum Biomarkers in Testicular Germ Cell Tumours: A Systematic Review. Eur Urol. 80: 456-466. https://doi.org/10.1016/j.eururo.2021.06.006
Syring I, Bartels J, Holdenrieder S, Kristiansen G, Müller SC, Ellinger J. 2015. Circulating serum miRNA (miR-367-3p, miR-371a-3p, miR-372-3p and miR-373-3p) as biomarkers in patients with testicular germ cell cancer. J Urol. 193: 331-337. https://doi.org/10.1016/j.juro.2014.07.010
Radtke A, Hennig F, Ikogho R, Hammel J, Anheuser P, Wülfing C, Belge G, Dieckmann KP. 2018. The Novel Biomarker of Germ Cell Tumours, Micro-RNA-371a-3p, Has a Very Rapid Decay in Patients with Clinical Stage 1. Urol Int. 100: 470-475. https://doi.org/10.1159/000488771
Nazzani S, Busico A, Bernasconi V, Bruniera M, Gianninò M, Rusconi D, Gualtieri J, Silvani C, Macchi A, Torelli T, Stagni S, Tesone A, Saitta C, Capone I, Cascella T, Lanocita R, Barella M, Paolini B, Perrone F, Albo G, Catanzaro MA, Biasoni D, Montanari E, Nicolai N; Italian Germ Cell Cancer Group. 2025. clinical evaluation of the role of miRNA 371 in small testicular masses. Results of the "S1STeM 371" Trial. Eur J Cancer. 223: 115494. https://doi.org/10.1016/j.ejca.2025.115494
Fankhauser CD, Wettstein MS, Christiansen AJ, Rothermundt C, Cathomas R, Kaufmann E, Sigg S, Templeton AJ, Hirschi-Blickenstorfer A, Lorch A, Gillessen S, Beyer J, Hermanns T. 2024. Cut-offs for relapse detection in men with stage I testicular germ cell tumors during active surveillance within a prospective multicentre cohort study using either raw or housekeeper normalized miR-371a-3p serum levels. Urol Oncol. 42: 455.e9-455.e13. https://doi.org/10.1016/j.urolonc.2024.07.013
Specifications: What makes the M731 test special
What makes the M371-Test so special?
- No health burden for the patient compared to other diagnostic methods
- Higher reliability due to very high sensitivity (90.1 %)
- Faster and more reliable differentiation of germ cell tumours from other diseases thanks to the high specificity of the M371-Test (94 %)
- Faster certainty through rapid normalisation of miR-371a-3p levels after treatment
- Improved efficiency of the healthcare system through faster diagnoses
- Uncomplicated integration into everyday clinical practice, as blood sampling is part of the routine examination
- Easy to establish in molecular biology laboratories
M371-Test - possible course of treatment
M371-Test - Specifications
- Ready-to-use reagents; all reagents required for the test are included
-
Validated for the following qPCR cyclers:
LightCycler® 480 II qPCR instrument (Roche Diagnostics) with 96-well heating block
cobas z 480 qPCR instrument (Roche Diagnostics) with 96-well heating block
LightCycler PRO qPCR instrument (Roche Diagnostics) with 96-well heating block
QuantStudio™ 5 Dx (Thermo Fisher Scientific) with 96-well heating block
QuantStudio™ 5 (Thermo Fisher Scientific) with 96-well heating block
AriaDx (Agilent Technologies) with 96-well heating block
CFX96 qPCR instrument (BioRad) with 96-well heating block -
Recommended RNA extraction kits:
miRNeasy Serum/Plasma Kit (50) (QIAGEN GmbH)
Maxwell RSC miRNA Plasma or Serum Kit (Promega GmbH) - Simple workflow
- Storage at -23 to -15°C
Further information on Liquid Biopsy
Liquid Biopsy
Liquid biopsy is a diagnostic procedure in which body fluids are analysed for substances that are specific to a disease. These substances are referred to as "biomarkers" or "markers".
Nucleic acids released by tumours, which can be present in the form of freely circulating tumour cells, free DNA (also known as cfDNA) or as exosomes (small vesicles that transport miRNA, for example), can be used as biomarkers for tumour diseases. Small amounts of blood, blood serum, blood plasma, urine or similar materials can be used as samples.
Advantages of the liquid biopsy procedure:
- Minimally invasive in contrast to other diagnostic methods
- The identification of tumour-specific molecules allows tumours to be detected at a particularly early stage
- Effective monitoring of therapy effects
- Earlier detection of recurrences in follow-up care
Further information on qPCR
The M371-Test is based on the method of quantitative real-time PCR (qPCR)
qPCR (Real Time-quantitative Polymerase Chain Reaction) is a molecular diagnostic method used to detect and quantify nucleic acids (DNA or RNA).
The polymerase chain reaction amplifies the nucleic acids to be detected (amplification). In qPCR, a fluorescence-labelled probe is also used. In the initial state, a molecule that is also bound to the probe prevents the fluorophores from being emitted. In the course of the PCR, the probe disintegrates due to the enzymatic activity of a DNA polymerase. In this state, the fluorescence is no longer absorbed and a fluorescence signal can be detected when excited by light. The more of the target nucleic acid is present in a sample, the earlier this signal can be measured. The time at which a threshold value is reached therefore provides information about the concentration of the target molecule in the sample.
The combination of qPCR with upstream pre-amplification increases the sensitivity of the M371-Test so that even the smallest amounts of tumour-specific miRNA can be detected. This method developed and optimised by mir|detect is protected by patent.

