
M371-Test | THE FUTURE OF TESTICULAR CANCER DIAGNOSTICS
Use our M371-Test for the diagnosis and follow-up of testicular cancer.
Minimally invasive test by blood draw
Superior sensitivity and reliability
Certified according to Regulation (EU) 2017/746 IVDR


Diagnostics
Biotracing
72,000 new cases per year worldwide
The current gold standard for the diagnosis and follow-up of testicular cancer is based on the serum tumor markers AFP, ß-hCG and LDH, ultrasound examinations and computerized tomography (CT). The sensitivity and specificity of the classic serum tumor markers are low, and a CT scan exposes patients to ionising radiation, which can cause secondary cancers.
The solution is called the M371-Test:
The liquid biopsy test detects the tumor-specific microRNA (miRNA) miR-371a-3p, which is released into the bloodstream by germ cell tumors. These tumors can therefore be detected with high sensitivity and specificity. In the follow-up care of testicular cancer patients, the M371-Test detects recurrences with a sensitivity of 100% and is therefore clearly superior to the classic markers.
Benefits
Biotracing
Advantages of the M371-Test
Greater security
By detecting the smallest amounts of tumor-specific miR-371a-3p in a blood sample, the M371-Test can be used in primary care to make a clear diagnosis more quickly and reliably.
Individualised therapy decisions
By reliably measuring the miR-371a-3p levels in the blood, the therapy can be customised for the affected patients. In follow-up care, the M371-Test can help to detect recurring tumors at an early stage and offer patients personalised treatment.
Cost reduction
The M371-Tests developed by mir|detect ensures that CT and MRI examinations can be further minimised or even completely eliminated.

Biotracing
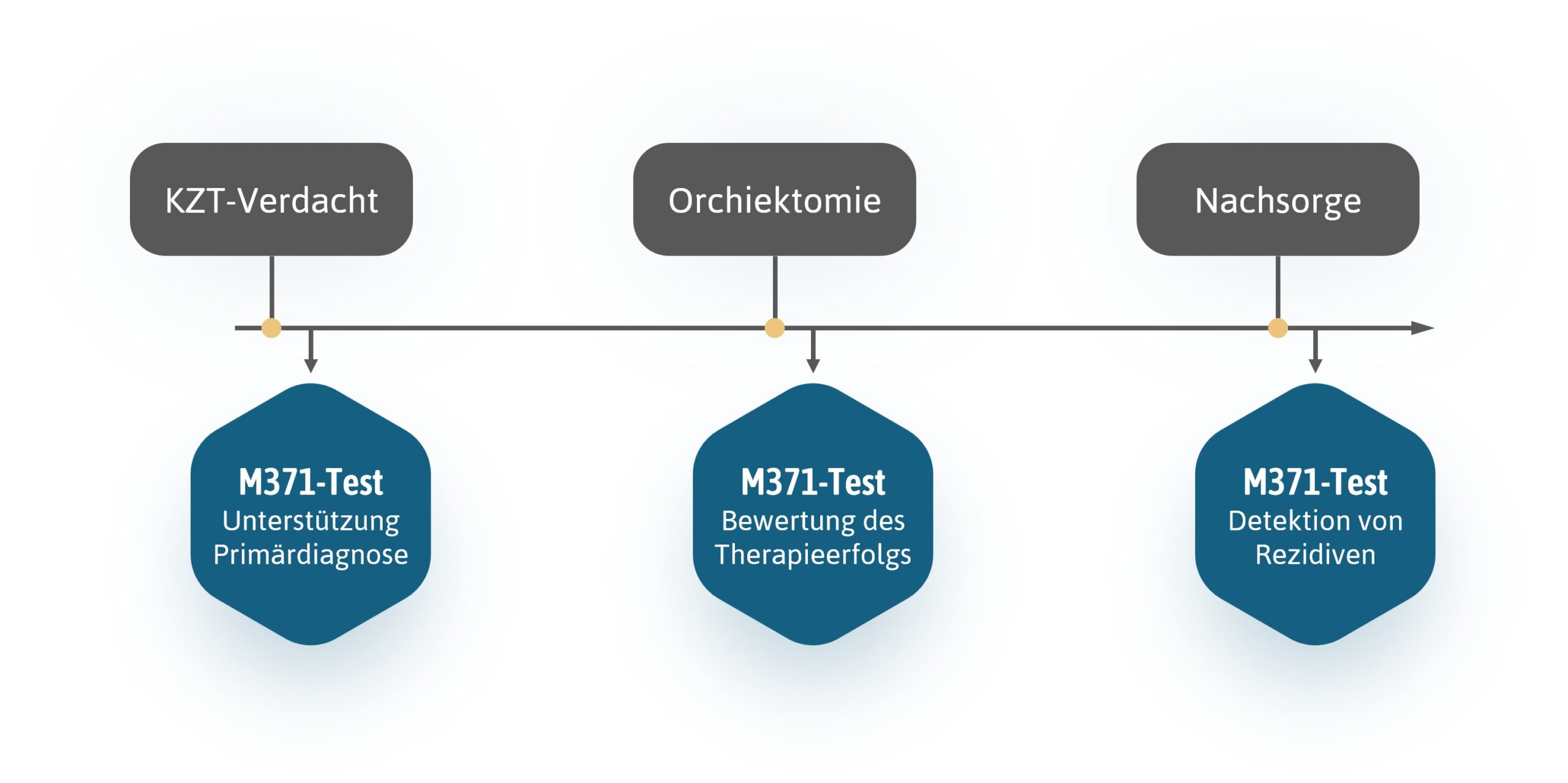
Application
The M371-Test in use
In our download area you can find the instructions for use and video tutorials on how to perform the M371-Test.

Introduction
Biotracing
How to use the M371-Test in your lab
Contact us
Receive introduction
Implement in lab

Latest news
Biotracing
Latest news
M371 Proficiency Check
QuantStudio 5 Dx
Distribution agreement with Gorea Plus
cobas z 480
LightCycler PRO & CFX96
Breakthrough Device Designation

Contact us
Biotracing
mir|detect: Biotechnology from Bremerhaven
Our mission:
Clarity & safety for testicular cancer.

